Acknowledgment for APICES collaboration in PRISMA-1 Study
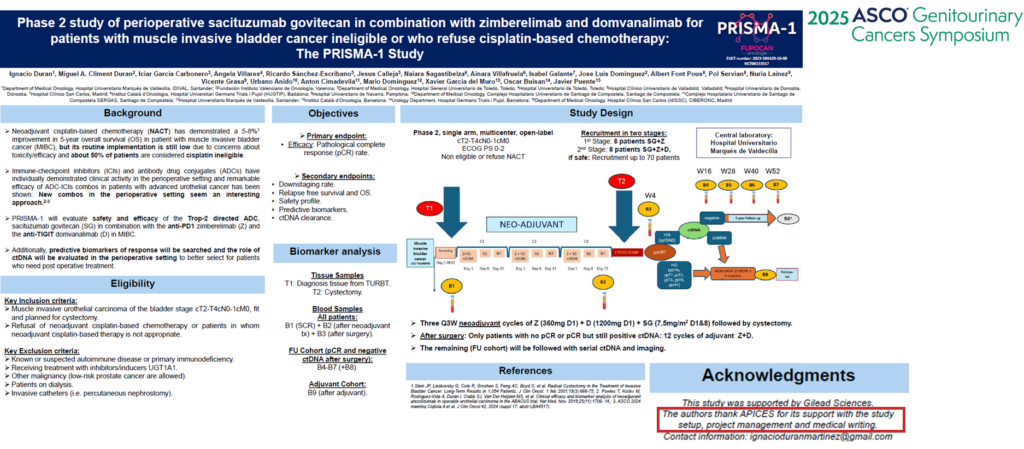
We want to share the acknowledgment that APICES has received in PRISMA-1 study, which is a phase II bladder cancer trial, published in the ASCO GU 2025 congress, in which APICES has collaborated in start-up, project management, and medical writing activities.
This means a great motivation to all the APICES team to give continuity to the implication we have in every project in which we collaborate. APICES is very proud of the recognition of our work, and we would like to thank Gilead, Dr. Ignacio Duran Martínez, and Fupocan for giving us the opportunity to appear in the acknowledgments of the publication and congratulate them and all the investigators for their project success.
This phase II clinical trial aims to evaluate perioperative immunotherapy combined with sacituzumab in patients with muscle-invasive bladder cancer ineligible or who refuse cisplatin-based chemotherapy.
You can find further information in the following link: Program Guide – ASCO Meeting Program Guide



