Over the past few years, real-world evidence (RWE) and health economics and outcomes research (HEOR) have shifted from supporting roles to central levers in how pharma designs trials, secures access, and manages the lifecycle of medicines. Regulators are formalising expectations for how RWE is generated and assessed; payers are raising the bar on economic and outcomes data; and digital health is expanding what “real-world” even means (1). At the same time, AI is fundamentally changing the way we curate, analyse, and interpret this evidence – bringing both opportunities and new responsibilities.
In this article we provide a practitioner’s view on where RWE and HEOR are heading, what leading organisations are actually doing with the data, how evidence is being generated differently, and how AI is reshaping the landscape – including the role specialised partners (CROs and RWE providers) now play.
RWE & HEOR today: from “supporting slides” to strategic assets
Regulatory and HTA use is still selective – but clearly growing. FDA and EMA now define and actively encourage RWE in specific use cases, with structured programs (e.g., FDA’s Advancing RWE Program and EMA’s RWE roadmap) and regular reporting on how RWE is used in decision-making. Recent analyses of EMA approvals between 2020-2023 show that fewer than 1 in 10 approvals used RWE in a meaningful way – but the trajectory is upward, and scrutiny of design and data quality is intense (2).
For medical affairs and RWE leaders, the centre of gravity is elsewhere:
- Informing treatment pathways and unmet need assessments
- Generating post-launch effectiveness and safety data in routine practice
- Supporting value dossiers and HTA submissions
- Understanding heterogeneity of treatment effect across sub-populations
- De-risking late-stage development and informing label expansion strategies
In many organisations, RWE and HEOR teams are now brought into cross-functional planning early – alongside clinical development, medical affairs, and market access – rather than being asked “to find some data” once a label or price is already in discussion.
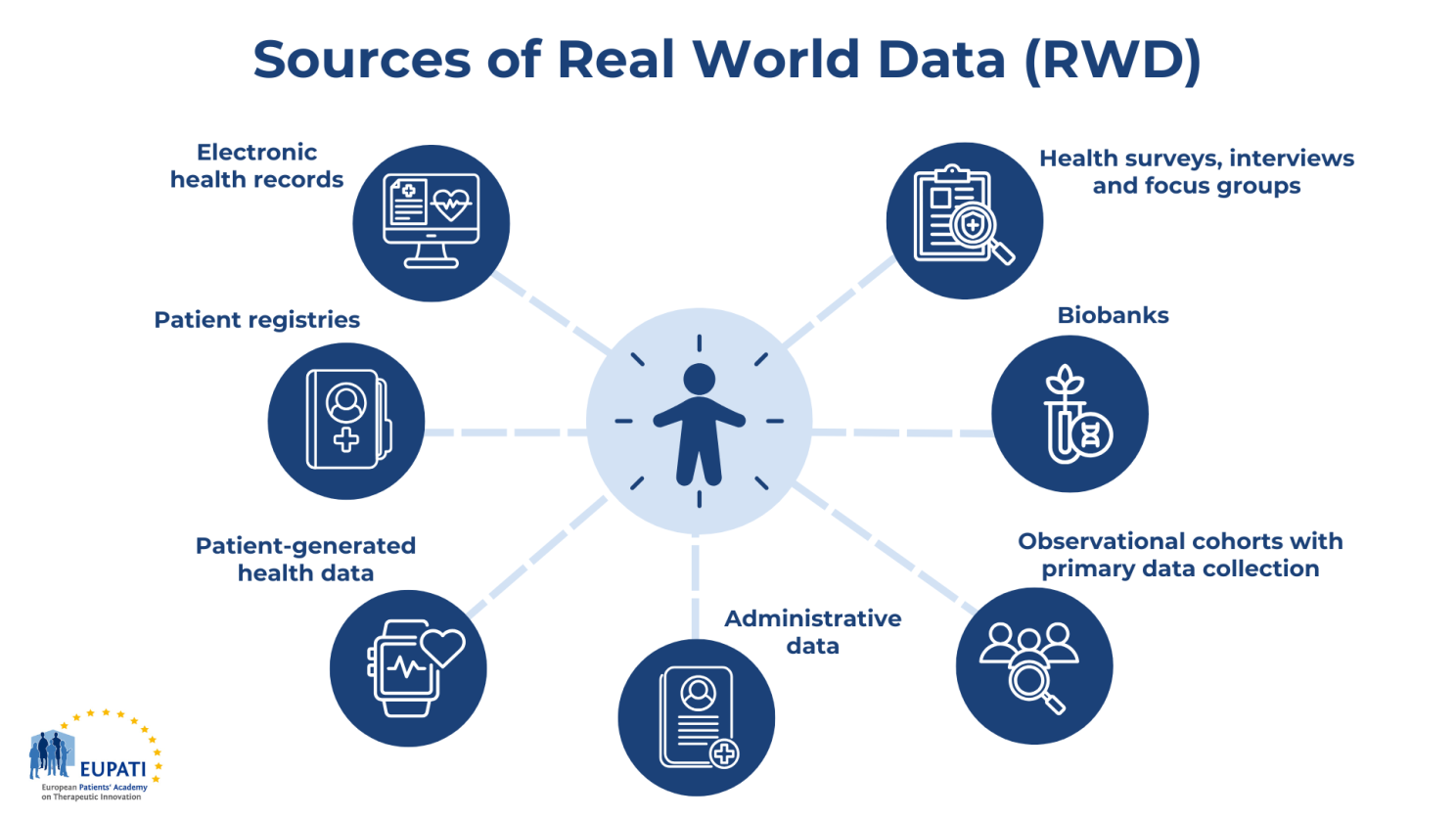
What companies are actually using RWE & HEOR for
Across the product lifecycle, we see a few recurring patterns:
Early development & trial design
- Epidemiology and burden of disease: quantifying incidence, prevalence, and disease trajectories to prioritise indications and inform target product profiles (3).
- Protocol optimisation: using historical RWD to simulate protocol eligibility criteria, estimate screen failure rates, and anticipate recruitment bottlenecks in different geographies.
- Site and country selection: leveraging EHR/claims data and registries to understand where eligible patients are actually seen and treated.
Late-phase, label and access
- External or hybrid control arms: supplementing or partially replacing conventional control groups with high-quality, carefully matched RWD – particularly in oncology, rare diseases, and single-arm trials (4).
- Long-term safety and effectiveness: registries, claims, and EHR-based cohorts help characterise persistence, adherence, and real-world outcomes beyond the trial timeframe.
- HTA and pricing: economic models drawing on RWE for inputs such as resource use, event rates, and utilities; local calibration of global models to reflect “how care is really delivered” in each health system (3).
Lifecycle management & medical affairs
- Sub-population insights: disaggregating outcomes by age, comorbidity, biomarkers, or socio-economic factors to identify groups who benefit more or less than the average trial patient.
- Treatment sequencing and patterns of care: mapping real-world lines of therapy, switching patterns, and guideline adherence; identifying gaps where educational or evidence-generation activities could meaningfully change practice (3).
- Value communication: furnishing field medical teams with robust, methodologically sound analyses – not just “real-world stories” – that stand up to scrutiny from clinicians and payers.
New ways evidence is generated: from static datasets to dynamic ecosystems
The most interesting trend is not simply more RWE, but more diverse, longitudinal and patient-centred data streams.
Digital health technologies and continuous data
Digital health technologies (DHTs) – from wearables and implantables to smartphone sensors and connected devices – now generate continuous, real-time measures of physiology and behaviour (activity, sleep, heart rate, glucose, arrhythmias, respiratory metrics, etc.) (5).
Regulators are increasingly explicit about how such mobile health (mHealth) data can be linked to EHRs, registries, and other sources to support representativeness and external validity, when captured in a structured and quality-assured manner.
For RWE and HEOR teams, this creates possibilities to:
- Use digital endpoints (e.g., gait speed, activity levels) as proxies or complements for traditional clinical outcomes
- Quantify treatment effect on daily life in ways that resonate with patients, clinicians, and payers
- Better understand adherence patterns in complex regimens (e.g., injectables, polypharmacy)
ePRO, eCOA and patient-centric data
Electronic patient-reported outcomes (ePRO), digital symptom diaries, and other eCOA tools are increasingly integrated into both interventional trials and post-authorisation studies. When designed well, they provide:
- Higher completion rates and more granular trajectories over time
- Improved capture of quality-of-life and treatment burden
- A richer basis for utility estimation and scenario analysis in economic models
“Next-generation” registries and pragmatic studies
We are also seeing a shift from static, paper-based registries to modular, technology-enabled registries that:
- Pull structured data from EHRs and prescribing systems
- Allow flexible, disease-agnostic modules to be added (e.g., PROs, specific biomarkers)
- Support nested sub-studies (e.g., pragmatic randomised comparisons or add-on sub-cohorts) without rebuilding the entire infrastructure
This is where the CRO lens naturally appears: setting up and maintaining these infrastructures – across multiple countries, data standards, and privacy regimes – is no longer a side-project. It requires coordinated expertise in clinical operations, data management, biostatistics, and real-world methodology.
AI as a force multiplier for RWE and HEOR
AI and machine learning (ML) are now embedded across the RWE/HEOR workflow – far beyond one-off “data science” pilots.
From unstructured notes to analysable cohorts
A large share of real-world data still sits in free-text clinical notes, radiology reports, pathology PDFs, and narrative discharge summaries. Modern NLP and large language models (LLMs) are increasingly used to:
- Extract diagnoses, staging, lines of therapy, response criteria, and toxicities from clinical narratives
- Build computable phenotypes and inclusion/exclusion algorithms at scale
- Harmonise terminology across institutions (e.g., mapping local phrasing to standard vocabularies such as SNOMED, ICD or MedDRA)
The best implementations use human-in-the-loop validation and robust audit trails to reach “regulatory-grade” RWE standards.
Smarter analytics for outcomes and economic evaluation
In HEOR, AI/ML techniques are being used to:
- Improve confounding adjustment and causal inference (e.g., high-dimensional propensity scores, targeted maximum likelihood)
- Develop predictive models of treatment response and adverse events, informing both personalised care and scenario analysis in cost-effectiveness models
- Explore complex heterogeneity of treatment effect across patient subgroups
- Optimise resource-use predictions and budget impact under different policy scenarios
Recent editorials also discuss how AI itself can be both subject and tool in economic evaluations: using AI in evidence generation, while also evaluating AI-based technologies with appropriate methodological safeguards (6).
Automating evidence synthesis and HEOR workflows
Generative AI is particularly visible in tasks that involve large volumes of text and structured data, such as (7):
- Screening and data extraction for systematic literature reviews and meta-analyses
- Drafting sections of reports, protocols, and value dossiers, using human experts for critical appraisal and finalisation
- Harmonising inputs and assumptions across multiple models or jurisdictions
The emphasis is gradually shifting from “can AI do this?” to “under what controls, and with what validation, can we safely incorporate AI into regulated HEOR and RWE workflows?”.
Quality, governance and trust: what “good” looks like
As expectations grow, so does scrutiny. Across regulators, HTA bodies, and scientific communities, a few themes consistently emerge:
- Fit-for-purpose data and design
- Robust data provenance and curation
- Validation and sensitivity analysis
- Interdisciplinary collaboration
This is precisely where many organisations lean on specialised partners with CRO DNA: to bring together clinical operations, RWD expertise, and HEOR capabilities in an integrated way – ensuring that pragmatic realities of care, regulatory expectations, and methodological rigour are aligned from the outset, not reconciled at the end.
Implications for Medical Affairs and RWE leaders
For medical affairs and RWE leaders in Pharma, a few practical implications stand out:
- Start with questions, not data assets. The temptation is to ask, “What can we do with this EHR/claims dataset?”. The more powerful approach is: “Which decisions will we need to make in the next 12–24 months, and what real-world evidence – generated under what assumptions – would materially de-risk those decisions?”
- Co-design RWE and HEOR strategies early. Bringing HEOR, RWE, and medical affairs together at protocol design and launch planning stages allows you to identify where pragmatic trials, registries, or hybrid RWE/clinical designs could provide multi-purpose evidence (regulatory, HTA, and medical) rather than siloed projects.
- Treat AI as an enabler, not a shortcut. AI can accelerate curation, analytics, and synthesis, but it does not remove the need for clear causal questions, carefully pre-specified analysis plans, and robust peer review. Governance, validation, and documentation will be decisive differentiators when AI-enabled evidence is scrutinised by regulators and HTA bodies.
- Invest in partnerships and infrastructure, not just projects. Sustainable impact comes from building interoperable data and analytics infrastructures – often jointly with hospitals, networks, and CRO/RWE partners – that can support multiple studies and indications over time, rather than one-off, bespoke solutions.
Closing thought
RWE and HEOR are no longer peripheral slide decks attached at the end of a submission. They are becoming integral to how we understand diseases, design trials, communicate value, and continuously learn from routine care.
The combination of increasingly rich real-world data, digital health technologies, and AI-enabled analytics offers enormous potential – but only if we match it with rigorous methods, transparent governance, and genuine collaboration between pharma, healthcare providers, patients, and specialised partners.
From a CRO perspective, that collaboration is where the real innovation happens: not in any single dataset or algorithm, but in the way we collectively turn messy, real-world signals into evidence that clinicians trust, regulators can rely on, and – most importantly – that ultimately improves outcomes for patients.
Planning a RWE/HEOR study in Europe? Let’s chat! Contact our business development team and schedule a call to know how APICES CRO handles the acceleration of study timelines and generation of valuable data according to plan.




